Analysis and Characterization of Hematopoietic Progenitor Cells from Fetal Bone Marrow, Adult Bone Marrow, Peripheral Blood, and Cord Blood
-
Post date: Jul 11, 2023 | Virginia, US
Abstract
Hematopoietic stem cell transplantation has been increasingly used to replace a defective hematopoietic system and to treat various genetic defects as well as malignant diseases. However, the limitations of conventional bone marrow transplantation have stimulated an intense interest in exploring the use of alternative sources of hematopoietic stem cells, including peripheral blood mononuclear cells (PBMC) and cord blood (CB). A major investigative effort of our laboratory has been focused on evaluating fetal bone marrow (FBM) for transplantation. The current study compares and characterizes the functional and phenotypic characteristics of FBM, CB, adult bone marrow (ABM), and PBMC by clonogenicity assays, immunogenicity, and the quantification of progenitor cells. There was a striking difference in the proportion of CD34+ cells in FBM, ABM, PBMC, and CB (24.6%, 2.1%, 0.5%, and 2.0%, respectively). The clonogenic potential, as measured by colony forming unit in culture (CFU-C) assay, was significantly higher in FBM when compared with ABM, PBMC, and CB (202.5, 73.5, 40.8, and 65.5 colonies/105 cells, respectively). There was a significant decrease in proliferative responsiveness in mixed lymphocyte reaction (MLR) assay of FBM and CB compared with ABM and PBMC. These observations indicate that each source of hematopoietic stem cells has different intrinsic properties closely correlated with ontogenetic age that is a vital determinant for phenotypic characteristics, lineage commitments, immunogenicity, and proliferative potentials.
Main
The reduced immunogenicity of fetal tissue makes it a useful donor source for transplantation. In addition, fetal tissues produce abundant trophic substances and growth factors, which promote cell growth and regeneration of damaged tissues and cells. The ability for rapid cellular growth, proliferation, differentiation, revascularization, and tissue repair has resulted in the successful use of fetal tissue transplantation in the treatment of various diseases, including Parkinson's disease, neural tissue injury, diabetes, and myocardial infarction as well as various genetic defects(1–8).
Hematopoietic stem cell transplantation has not only been increasingly used for correction of abnormal hematopoiesis in patients with malignant and nonmalignant diseases but also has made high dose chemotherapy possible for the treatment of many solid malignancies. Historically, the adult bone marrow has been the major source of hematopoietic stem cells. However, the shortage of availability of donors for allogeneic bone marrow transplantation and the risk of malignant contamination in autologous bone marrow have stimulated a search for alternative sources of stem cells. One of the prime sources has been PBMC, which offers several advantages including rapid hematopoietic and immunologic reconstitution and frequently lower risks of contamination with tumor cells compared with bone marrow grafts(9,10). Similarly, CB is being used as an alternative source and offers the advantages of easy accessibility and a good hematopoietic cellular yield, which is equivalent to that of ABM(11–14). Although there are variable levels of maternal cell contamination observed in CB that may be associated with GVHD, this has not posed any major clinical problem(15,16). However, the relatively small number of primitive stem cells and large number of committed progenitors per donor in CB may affect its therapeutic efficacy. On the other hand, transplantation of fetal tissue in animal models and in human experimental treatments with fetal liver, thymus, spleen, and bone marrow have shown distinct advantages over adult tissues including lowered immunogenicity and higher percent of primitive cells. Fetus-derived stem cells potentially remove the problems of tissue typing and also have a high capacity to differentiate into the complete repertoire of erythroid, myeloid, and lymphoid lineages.
The present studies were performed to compare the phenotypic characteristics of FBM, harvested from second trimester of lost pregnancies, with ABM, PBMC, and CB. In addition, the clonogenic potential and immunogenicity of each of these cell sources were evaluated to determine their suitability for transplantation.
METHODS
Cell sources and processing. FBM (n = 8) was individually harvested from fetuses of lost pregnancies in the second trimester (18 to 20 wk gestation). FBM cells were collected from femurs, tibias, vertebral columns, pelvic bones, and scapulae, which were harvested en bloc, dissected aseptically, and cut into small pieces. After removing the cortex, the marrow-rich cancellous portion was minced with a homogenizer. In all cases, the tissues were collected after informed consent was obtained. Normal ABM (n = 8) was obtained from normal allogeneic bone marrow donors according to standard techniques developed earlier(17). The volume of each aspiration was limited to < 5 mL to minimize the contamination of peripheral blood with the harvested marrow(18,19). ABM cells were procured by flushing marrow collection bags and filter screens used in harvesting marrow from normal allogeneic donors. Recombinant human granulocyte-colony stimulating factor (rhG-CSF) mobilized PBMC (n = 8) were collected from normal volunteer donors. CB (n = 12) was obtained immediately after delivery by draining samples from the umbilical cord vein into sterile collection tubes containing preservative-free heparin. PBMC and CB were diluted to 1:3 with PBS. The low-density mononuclear cells were separated by Ficoll-Hypaque (Atlanta Biologicals, GA) density centrifugation and washed twice with PBS. Cell yield and viability were determined by trypan blue dye exclusion assay. The logistics of human donors were handled according to Department of Health and Human Services/National Institutes of Health guidelines on protection of human subjects, with the authorization of the Georgetown University Medical School IRB Committee.
Phenotypic analysis. Two-color immunofluorescent staining was used to analyze the phenotypic characteristics of mononuclear cells. Mononuclear cells were resuspended in PBS containing 0.3% BSA, 0.25% human immune globulin, and 0.3% sodium azide at a concentration of 106/100 µL and incubated for 15 min at 25°C. An aliquot of 5 × 105 cells was removed and stained with monoclonal antibodies directed against various human lymphocyte and/or myeloid antigens. Monoclonal antibody preparations directed against the following surface markers were used: CD34, CD38, CD3, CD16, CD19, CD56, and HLA-DR (Becton Dickinson, San Jose, CA). Cell analysis was performed on FACStarplus (Becton Dickinson). The data were analyzed with Reproman and Lysys software.
Clonogenic cultures. Mononuclear cells from all sources were added to semisolid media containing 0.8% of methylcellulose (HCC 4230; StemCell Technologies, Inc., Vancouver, Canada) supplemented with erythropoietin (Epo, 2 u/mL) and granulocyte/macrophage colony stimulating factor (GM-CSF, 20 ng/mL). The cells were plated at a concentration of 1 × 105/mL and for each group, two 1-mL cultures were set up in 35-mm Petri dishes. Plates were incubated at 37°C in a humidified atmosphere of 5% CO2 for 2 weeks. The number of CFU-C was scored on d 14 with a colony consisting of 40 or more cells.
Mixed lymphocyte reactions (MLR). MLRs were conducted by culturing 1 × 105 mononuclear cells with equal numbers of irradiated autologous or allogeneic bone marrow or blood cells in 96 well round-bottomed microtiter plates. The stimulator cells were irradiated with 1500 rads with a JL Shepard Cs-137 Irradiator. All cultures were performed in triplicate in 0.2 mL of RPMI 1640 supplemented with 10% human AB serum. Cultures were maintained in a 37°C and 5% CO2 humidified incubator for 5 d. DNA synthesis was measured by pulsing with 1 µCi of 3H-thymidine during the final 18 h of the culture. Cultures were harvested onto glass fiber filter paper using a PHD™ cell harvester (Cambridge Technology Inc., MA) and 3H-thymidine incorporation was determined by liquid scintillation counting.
Statistical analysis. Each of the markers was tested independently using an analysis of variance (ANOVA). In order to achieve normality and equal variance assumptions for the ANOVA, the marker expression data were transformed using natural logs. Differences in the collection types (FBM, CM, PBMC, and ABM) were tested using Tukey's multiple comparison method(20) to control the overall significance level at 0.05 for each marker. The colony formation data were tested in a similar manner, but the data were transformed using the square root, which is a standard transformation instrument for assessment of data to achieve normally distributed values. The overall significance level was 0.01 for the multiple comparison method to control for using the combined data. Where applicable, simple correlations were performed.
RESULTS
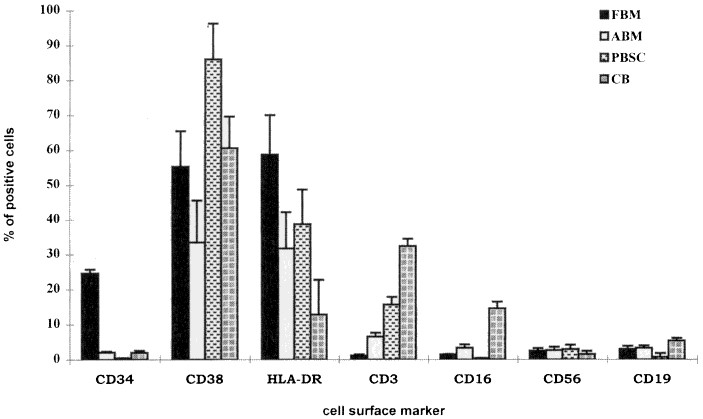
Proportion of hematopoietic progenitors. The quantitative yield of mononuclear cells from each single fetus was approximately 1-3 × 108 and was dependent on the age and the available number of fetal bones. The most striking and unique characteristics of FBM were the number of CD34+ cells in the mononuclear cells after Ficoll separation. The percentage of CD34+ cells in FBM ranged from 14.8 to 30.7 (mean 24.6 ± 3.5) which was significantly higher when compared with the other three sources, i.e. ABM, PBMC, and CB (Fig. 1). In comparison, the percentages of CD34+ cells in ABM and CB were equivalent, ranging from 1.3-3.4% and 1.2-2.9%, respectively. PBMC had the lowest number of CD34+ cells (0.2-0.8%). Because the majority of the CD34+ cells are committed to either the hematopoietic or stromal cell lineages, we further compared the most common lineage associated antigens, CD38 and HLA-DR, expression within the fraction of CD34+ cells. As shown in Table 1 and 2, FBM had the highest number of CD34+/38- and CD34+/HLA-DR- cells (2.11 ± 0.21% and 3.12 ± 0.19%, respectively). The number of CD34+ cells was statistically analyzed by using ANOVA (Table 2). FBM had the highest log expression (3.13), CB and ABM were better than PBMC, but were not different from each other (mean log expression: 1.09, 1.11, and 0.376, respectively). For the CD34+/DR- and CD34+/CD38- combination, FBM (1.10) remained the highest. PBMC and ABM were better than CB, but they were not significantly different from each other.
Read the full article here.
From the same category
Analysis and Characterization of Hematopoietic Progenitor Cells from Fetal Bone Marrow, Adult Bone Marrow, Peripheral Blood, and Cord Blood
-
Post date: Jul 11, 2023 | Virginia, US
Abstract
Hematopoietic stem cell transplantation has been increasingly used to replace a defective hematopoietic system and to treat various genetic defects as well as malignant diseases. However, the limitations of conventional bone marrow transplantation have stimulated an intense interest in exploring the use of alternative sources of hematopoietic stem cells, including peripheral blood mononuclear cells (PBMC) and cord blood (CB). A major investigative effort of our laboratory has been focused on evaluating fetal bone marrow (FBM) for transplantation. The current study compares and characterizes the functional and phenotypic characteristics of FBM, CB, adult bone marrow (ABM), and PBMC by clonogenicity assays, immunogenicity, and the quantification of progenitor cells. There was a striking difference in the proportion of CD34+ cells in FBM, ABM, PBMC, and CB (24.6%, 2.1%, 0.5%, and 2.0%, respectively). The clonogenic potential, as measured by colony forming unit in culture (CFU-C) assay, was significantly higher in FBM when compared with ABM, PBMC, and CB (202.5, 73.5, 40.8, and 65.5 colonies/105 cells, respectively). There was a significant decrease in proliferative responsiveness in mixed lymphocyte reaction (MLR) assay of FBM and CB compared with ABM and PBMC. These observations indicate that each source of hematopoietic stem cells has different intrinsic properties closely correlated with ontogenetic age that is a vital determinant for phenotypic characteristics, lineage commitments, immunogenicity, and proliferative potentials.
Main
The reduced immunogenicity of fetal tissue makes it a useful donor source for transplantation. In addition, fetal tissues produce abundant trophic substances and growth factors, which promote cell growth and regeneration of damaged tissues and cells. The ability for rapid cellular growth, proliferation, differentiation, revascularization, and tissue repair has resulted in the successful use of fetal tissue transplantation in the treatment of various diseases, including Parkinson's disease, neural tissue injury, diabetes, and myocardial infarction as well as various genetic defects(1–8).
Hematopoietic stem cell transplantation has not only been increasingly used for correction of abnormal hematopoiesis in patients with malignant and nonmalignant diseases but also has made high dose chemotherapy possible for the treatment of many solid malignancies. Historically, the adult bone marrow has been the major source of hematopoietic stem cells. However, the shortage of availability of donors for allogeneic bone marrow transplantation and the risk of malignant contamination in autologous bone marrow have stimulated a search for alternative sources of stem cells. One of the prime sources has been PBMC, which offers several advantages including rapid hematopoietic and immunologic reconstitution and frequently lower risks of contamination with tumor cells compared with bone marrow grafts(9,10). Similarly, CB is being used as an alternative source and offers the advantages of easy accessibility and a good hematopoietic cellular yield, which is equivalent to that of ABM(11–14). Although there are variable levels of maternal cell contamination observed in CB that may be associated with GVHD, this has not posed any major clinical problem(15,16). However, the relatively small number of primitive stem cells and large number of committed progenitors per donor in CB may affect its therapeutic efficacy. On the other hand, transplantation of fetal tissue in animal models and in human experimental treatments with fetal liver, thymus, spleen, and bone marrow have shown distinct advantages over adult tissues including lowered immunogenicity and higher percent of primitive cells. Fetus-derived stem cells potentially remove the problems of tissue typing and also have a high capacity to differentiate into the complete repertoire of erythroid, myeloid, and lymphoid lineages.
The present studies were performed to compare the phenotypic characteristics of FBM, harvested from second trimester of lost pregnancies, with ABM, PBMC, and CB. In addition, the clonogenic potential and immunogenicity of each of these cell sources were evaluated to determine their suitability for transplantation.
METHODS
Cell sources and processing. FBM (n = 8) was individually harvested from fetuses of lost pregnancies in the second trimester (18 to 20 wk gestation). FBM cells were collected from femurs, tibias, vertebral columns, pelvic bones, and scapulae, which were harvested en bloc, dissected aseptically, and cut into small pieces. After removing the cortex, the marrow-rich cancellous portion was minced with a homogenizer. In all cases, the tissues were collected after informed consent was obtained. Normal ABM (n = 8) was obtained from normal allogeneic bone marrow donors according to standard techniques developed earlier(17). The volume of each aspiration was limited to < 5 mL to minimize the contamination of peripheral blood with the harvested marrow(18,19). ABM cells were procured by flushing marrow collection bags and filter screens used in harvesting marrow from normal allogeneic donors. Recombinant human granulocyte-colony stimulating factor (rhG-CSF) mobilized PBMC (n = 8) were collected from normal volunteer donors. CB (n = 12) was obtained immediately after delivery by draining samples from the umbilical cord vein into sterile collection tubes containing preservative-free heparin. PBMC and CB were diluted to 1:3 with PBS. The low-density mononuclear cells were separated by Ficoll-Hypaque (Atlanta Biologicals, GA) density centrifugation and washed twice with PBS. Cell yield and viability were determined by trypan blue dye exclusion assay. The logistics of human donors were handled according to Department of Health and Human Services/National Institutes of Health guidelines on protection of human subjects, with the authorization of the Georgetown University Medical School IRB Committee.
Phenotypic analysis. Two-color immunofluorescent staining was used to analyze the phenotypic characteristics of mononuclear cells. Mononuclear cells were resuspended in PBS containing 0.3% BSA, 0.25% human immune globulin, and 0.3% sodium azide at a concentration of 106/100 µL and incubated for 15 min at 25°C. An aliquot of 5 × 105 cells was removed and stained with monoclonal antibodies directed against various human lymphocyte and/or myeloid antigens. Monoclonal antibody preparations directed against the following surface markers were used: CD34, CD38, CD3, CD16, CD19, CD56, and HLA-DR (Becton Dickinson, San Jose, CA). Cell analysis was performed on FACStarplus (Becton Dickinson). The data were analyzed with Reproman and Lysys software.
Clonogenic cultures. Mononuclear cells from all sources were added to semisolid media containing 0.8% of methylcellulose (HCC 4230; StemCell Technologies, Inc., Vancouver, Canada) supplemented with erythropoietin (Epo, 2 u/mL) and granulocyte/macrophage colony stimulating factor (GM-CSF, 20 ng/mL). The cells were plated at a concentration of 1 × 105/mL and for each group, two 1-mL cultures were set up in 35-mm Petri dishes. Plates were incubated at 37°C in a humidified atmosphere of 5% CO2 for 2 weeks. The number of CFU-C was scored on d 14 with a colony consisting of 40 or more cells.
Mixed lymphocyte reactions (MLR). MLRs were conducted by culturing 1 × 105 mononuclear cells with equal numbers of irradiated autologous or allogeneic bone marrow or blood cells in 96 well round-bottomed microtiter plates. The stimulator cells were irradiated with 1500 rads with a JL Shepard Cs-137 Irradiator. All cultures were performed in triplicate in 0.2 mL of RPMI 1640 supplemented with 10% human AB serum. Cultures were maintained in a 37°C and 5% CO2 humidified incubator for 5 d. DNA synthesis was measured by pulsing with 1 µCi of 3H-thymidine during the final 18 h of the culture. Cultures were harvested onto glass fiber filter paper using a PHD™ cell harvester (Cambridge Technology Inc., MA) and 3H-thymidine incorporation was determined by liquid scintillation counting.
Statistical analysis. Each of the markers was tested independently using an analysis of variance (ANOVA). In order to achieve normality and equal variance assumptions for the ANOVA, the marker expression data were transformed using natural logs. Differences in the collection types (FBM, CM, PBMC, and ABM) were tested using Tukey's multiple comparison method(20) to control the overall significance level at 0.05 for each marker. The colony formation data were tested in a similar manner, but the data were transformed using the square root, which is a standard transformation instrument for assessment of data to achieve normally distributed values. The overall significance level was 0.01 for the multiple comparison method to control for using the combined data. Where applicable, simple correlations were performed.
RESULTS
Proportion of hematopoietic progenitors. The quantitative yield of mononuclear cells from each single fetus was approximately 1-3 × 108 and was dependent on the age and the available number of fetal bones. The most striking and unique characteristics of FBM were the number of CD34+ cells in the mononuclear cells after Ficoll separation. The percentage of CD34+ cells in FBM ranged from 14.8 to 30.7 (mean 24.6 ± 3.5) which was significantly higher when compared with the other three sources, i.e. ABM, PBMC, and CB (Fig. 1). In comparison, the percentages of CD34+ cells in ABM and CB were equivalent, ranging from 1.3-3.4% and 1.2-2.9%, respectively. PBMC had the lowest number of CD34+ cells (0.2-0.8%). Because the majority of the CD34+ cells are committed to either the hematopoietic or stromal cell lineages, we further compared the most common lineage associated antigens, CD38 and HLA-DR, expression within the fraction of CD34+ cells. As shown in Table 1 and 2, FBM had the highest number of CD34+/38- and CD34+/HLA-DR- cells (2.11 ± 0.21% and 3.12 ± 0.19%, respectively). The number of CD34+ cells was statistically analyzed by using ANOVA (Table 2). FBM had the highest log expression (3.13), CB and ABM were better than PBMC, but were not different from each other (mean log expression: 1.09, 1.11, and 0.376, respectively). For the CD34+/DR- and CD34+/CD38- combination, FBM (1.10) remained the highest. PBMC and ABM were better than CB, but they were not significantly different from each other.
Read the full article here.